简介
临床特征。亨廷顿病(HD)是一种进行性运动,认知和精神障碍的疾病。发病的平均年龄为35至44岁,中位生存时间为发病后的15至18年。
诊断/测试。HD的诊断取决于阳性家族史,特征性临床发现以及在HTT中检测到36个或更多CAG三核苷酸重复序列的扩增。
管理。表型的治疗:药物治疗,包括典型的精神安定药(氟哌啶醇),非典型神经安定药(奥氮平),苯二氮卓或多巴胺耗竭剂丁苯那嗪,用于舞蹈运动。抗帕金森氏症的运动不足和僵硬;用于精神疾病(抑郁症,精神病性症状,伤人)的精神药物或某些类型的抗癫痫药;丙戊酸用于肌阵挛性运动亢进。支持性护理,注意护理需求,饮食摄入,专用设备以及获得州和联邦福利的资格。
预防继发并发症:注意需要长期支持治疗的人的常见潜在并发症以及与药物治疗相关的副作用。
监视:定期评估舞蹈病的外观和严重程度,僵硬,步态问题,抑郁,行为改变和认知能力下降;使用行为观察量表亨廷顿(BOSH)和统一高清评分量表(UHDRS)对功能能力进行常规评估。
避免接触的试剂/情况:含左旋多巴的化合物(可能加重舞蹈病),饮酒,吸烟。
其他:父母有HD的儿童和青少年可能会从当地的HD支持小组中获得教育材料和心理支持,从而受益。
遗传咨询。HD以 常染色体显性遗传的方式遗传。具有致病性变异的个体的后代有50%的机会遗传引起疾病的等位基因。可以对有风险的无症状成人进行预测性测试,但由于目前尚无法治愈该疾病,因此需要仔细考虑(包括测试前和测试后的 遗传咨询)。但是,有风险的无症状个体可能有资格参加临床试验。预测性测试不适用于年龄在18岁以下的无症状高危人群。可以通过 分子遗传学检测进行产前检查。
诊断
拟诊
具有以下任何一项的个人都应怀疑患有亨廷顿病(HD):
- 具有舞蹈病的进行性运动障碍。 主动运动也可能受到影响。
- 精神障碍,包括认知能力下降,人格改变和/或抑郁
- 家族史与 常染色体显性遗传一致
注意:运动障碍,认知障碍和精神障碍的出现和顺序在 HD中可能会有所不同(请参阅Clinical Description)。
建立诊断
通过分子遗传学检测鉴定HTT中CAG三核苷酸重复的杂合的扩展,可在具有HD的临床体征和症状的先证者中确认HD的诊断(参见 Table 1)。
等位基因大小。 所有患有HD的个体的HTT 外显子1中编码谷氨酰胺氨基酸的CAG三核苷酸重复序列的数量都有增加。
- 正常等位基因。 26个或更少的CAG三核苷酸重复序列。
- 过渡区等位基因。 27-35 CAG三核苷酸重复。 等位基因在此范围内的个体没有发展为HD症状的风险,但是由于CAG的不稳定性,孩子可能有导致等位基因在HD范围内的风险[Semaka et al 2006]。 胚系CAG扩展的风险评估已建立 [Semaka et al 2013b, Semaka & Hayden 2014].
- 引起HD等位基因。 36个或更多的CAG三核苷酸重复序列。 有引起HD等位基因的人一生中有HD的风险。 引起HD等位基因进一步分类为:
- HD致病等位基因的外显率降低。 36-39 CAG三核苷酸重复。 等位基因在此范围内的个体有患HD的风险,但可能不会出现症状。 具有此范围内CAG重复的老年人无症状者是常见的 [Kay et al 2016]。.
- 导致HD的等位基因全外显。 40个或更多的CAG三核苷酸重复序列。 假定正常寿命,这种大小的等位基因与HD的发生具有确定性。
分子遗传学测试方法包括针对HTT中CAG重复序列大小的目标测试。
Table 1.
Table 1
亨廷顿病分子遗传学检测
| 基因 1 | 测试方法 | 具有致病变异的先证者所占比例 2 可通过此方法检测 |
|---|---|---|
| HTT | Targeted analysis 3 | 100% 4 |
- 1.
参见 Table A. Genes and Databases 。 染色体 位点和蛋白质。
- 2.
有关在该 基因中检测到的等位基因变异的信息,请参见 Molecular Genetics。
- 3.
检测CAG三核苷酸重复数。 基于PCR的方法可检测多达115个CAG重复序列的等位基因[Potter et al 2004, Levin et al 2006]。 其他方法有时可能对识别与青少年期HD相关的大型CAG重复片段或确认通过常规PCR分析获得的表观 纯合性 基因型有用。 其他方法可能包括三重重复引物PCR[Jama et al 2013]或 Southern blot 分析 [Bean & Bayrak-Toydemir 2014]。 有关与HD相关的预测测试的更多考虑,请参见Benjamin et al [1994]。
- 4.
注意:有关与HD预测性基因测试有关的综合建议,请参见遗传咨询, Related Genetic Counseling Issues and MacLeod et al [2013].
临床特征
临床表现
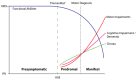
在亨廷顿病(HD)的前驱阶段,个体的运动技能,认知和性格可能会发生细微变化(Figure 1) [Tabrizi et al 2013, Ross et al 2014, Liu et al 2015]。 这些细微的变化可以在明显的HD临床发作之前的15-20年内发生。
Reilmann et al [2014] 前驱和典型HD诊断标准指南; 参见 Table 2.。该表可用于将个体分为不同的诊断类别,这些诊断类别可能随时间推移而具有临床管理意义。 例如,对症状前和前驱性HD的了解可以进行预防性(而非对症治疗)治疗。 注意分类系统中遗传确认的 HD明显区别。
Table 2.
亨廷顿病诊断类别
| HD分类 | HD 体征/症状 | |
|---|---|---|
| 经过基因确认 | 未经基因确认 | |
症状前HD:HD,经基因证实,症状前 |
临床上有HD风险的人:未经基因证实的HD,有临床风险 |
|
前驱HD:HD,经过基因确认,前驱 |
临床前驱HD:HD,未经基因证实,临床前驱 |
|
HD表现:HD,经过基因确认,有表现 | HD的临床表现:HD,未经基因证实,临床表现 1 |
|
改编自 Reilmann et al [2014]; 经许可使用
DCL = UHDRS评分量表的诊断置信度; HD =亨廷顿病; TFC =总功能容量
- 1.
需要运动DCL = 4,加上认知变化
HD的平均发病年龄约为45岁[Bates et al 2015]。约三分之二的受累的首先表现出神经系统表现。其他人出现精神病学改变。在诊断后的早期阶段,表现包括眼球运动的细微变化,协调性,轻微的非自愿运动,心理计划困难以及情绪低落或烦躁(见Table 3)。受影响的个体通常能够执行其大部分日常活动并继续工作[Ross et al 2014, Bates et al 2015]。
在大约25%的HD患者中,发病会推迟到50岁以后,有些会推迟到70岁以后。这些人患有舞蹈症,步态障碍和吞咽困难,但与典型症状相比,病程更长,更良性。
在下一阶段,舞蹈病变得更加突出,主动活动变得越来越困难,构音困难和吞咽困难恶化。尽管大多数人仍然能够维持相当程度的个人独立性,但大多数人被迫放弃工作并越来越依赖他人寻求帮助。损害通常是相当大的,有时会间歇性爆发攻击性行为和社会禁忌行为。
在HD的晚期,运动障碍变得很严重,个体通常完全依赖,沉默和尿失禁。发病后的中位生存时间为15至18年(范围:5至> 25年)。平均死亡年龄为54至55岁 [Bates et al 2015]。
Table 3.
HD的临床体征和症状发作
| HD临床表现 | |
|---|---|
| 早期 | 笨拙 |
| 激动 | |
| 易怒 | |
| 冷漠 | |
| 焦虑 | |
偏执 | |
| 妄想 | |
| 幻觉 | |
| 眼睛运动异常 | |
| 抑郁 | |
| 嗅觉功能障碍 | |
| 中期 | 肌张力异常 |
| 不自主运动 | |
| 平衡障碍&行走困难 | |
| 舞蹈症,扭曲 &扭动动作,抽搐,蹒跚,摇摆,步态脱节(看起来像陶醉) | |
| 精细动作困难 | |
| 主动运动缓慢,难以启动 | |
| 无法控制速度 &移动力 | |
| 反应时间慢 | |
| 虚弱 | |
| 体重减轻 | |
| 构音困难 | |
| 固执 | |
| 晚期 | 僵硬 |
| 运动迟缓(启动&持续运动困难) | |
| 严重的舞蹈病(少见) | |
| 体重明显减轻 | |
| 不能走路 | |
| 无法说话 | |
| 吞咽问题,窒息危险 | |
| 无法照顾自己 | |
运动异常。患有HD的人会发生非自主和自主运动的障碍。舞蹈病是一种由非重复性,非周期性的四肢,面部或躯干抽动组成的非自主运动障碍,是该疾病的主要症状。舞蹈病存在于90%以上的个体中,并且通常在头十年中病情严重程度会增加。舞蹈动作在清醒时间内持续存在,不能受控制,并且由于压力而恶化。
随着疾病持续时间的增加,会发生其他非自主运动,例如运动迟缓,僵硬和肌张力障碍。自主运动功能受损是早期迹象。受影响的个人及其家人在日常的日常活动中描述笨拙。运动速度,精细控制和步态都会受到影响。动眼障碍发生较早,并逐渐恶化。在多达75%的有症状个体中,启动眼球扫视,眼球扫视过慢和视线凝视困难 [Blekher et al 2006, Golding et al 2006]。构音障碍发生较早并且很常见。吞咽困难发生在晚期。反射亢进在90%的个体中较早发生,而肌阵挛和伸肌足底反应迟顿较不常发生。
认知异常。在所有患有HD的个体中,认知能力总体逐步下降。认知变化包括健忘,思维过程缓慢,视觉空间能力受损以及操纵所学知识的能力受损。多项研究已经在运动症状发作之前发现了微妙但确定的认知缺陷[Bourne et al 2006, Montoya et al 2006, Paulsen et al 2008, Tabrizi et al 2009, Rupp et al 2010]。最初的变化通常涉及灵活性的丧失和执行功能的损害,例如计划和组织活动能力损害。
记忆缺陷伴随信息恢复损较早发生,但是口头提示,启动和足够的时间可能导致部分或正确的回忆。在疾病的早期,HD的记忆障碍通常不如阿尔茨海默氏病严重。
患有HD的人的整体认知和行为综合症与额颞痴呆更相似,而不同于阿尔茨海默氏病。注意力和集中力受损很早就涉及到了[Peinemann et al 2005],容易注意力分散。语言功能相对保留,但在后期阶段,语法复杂性降低,皮层语音异常,语法错误和字词查找困难。
神经心理学测试显示视觉空间能力受损,尤其是在疾病晚期。缺乏意识,尤其是缺乏对自身残疾的意识,这很普遍 [Ho et al 2006, Bates et al 2015]。
精神障碍。患有HD的个体会出现明显的性格变化,情感性精神病或精神分裂症性精神病[Rosenblatt 2007]。在HD发作之前,他们往往在抑郁,敌对,强迫症,焦虑和精神病等方面得分较高[Duff et al 2007]。与进行性认知和运动障碍不同,精神病学变化往往不会随着疾病的严重程度而发展[Epping et al 2016]。行为失常是经常发生的,例如间歇性躁狂,冷漠,攻击性,酗酒,性功能障碍和偏离以及食欲增加。妄想症,通常是偏执狂,很常见。幻觉不那么常见。
抑郁和自杀风险。临床前驱和有症状患者的抑郁症发病率是普通人群的两倍以上 [Paulsen et al 2005b, Marshall et al 2007]。 HD的抑郁症病因尚不清楚。这可能是该疾病的病理性后果,而非心理后果[Slaughter et al 2001, Pouladi et al 2009]。自杀和自杀观念在HD患者中很常见,但其发病率随疾病进程和预测性检测结果而变化 [Larsson et al 2006, Robins Wahlin 2007, van Duijn et al 2018]。自杀风险的关键时期被发现是在接受诊断之前,之后是受累者的自理能力丧失[Baliko et al 2004, Paulsen et al 2005a, Eddy et al 2016]。
其他。患有HD的人的体重指数往往低于对照组[Pratley et al 2000, Stoy & McKay 2000, Djoussé et al 2002, Robbins et al 2006],这可能与新陈代谢的改变有关[Duan et al 2014],且能代表临床进展 [van der Burg et al 2017]。患有HD的人也表现出胆固醇代谢紊乱[Valenza & Cattaneo 2006, Wang et al 2014]。患有HD的人通常表现出食欲和能量消耗增加[Pratley et al 2000, Trejo et al 2004, Gaba et al 2005]。
患有HD的个体的睡眠和昼夜节律受到干扰 [Goodman & Barker 2010, Morton 2013],可能是由于下丘脑功能障碍 [Goodman & Barker 2010, Morton 2013]和/或褪黑激素分泌改变 [Kalliolia et al 2014]。失眠和白天也可能出现失眠,尽管这更常见于精神病的变化,抑郁或舞蹈症 [Videnovic et al 2009]。
神经病理学。 HD的主要神经病理学特征是尾状和壳状核以及大脑皮层中神经元的变性 [Waldvogel et al 2015]。基底神经节运动控制间接途径中含有脑啡肽的中棘神经元的变性为舞蹈病提供了神经生物学基础 [Galvan et al 2012]。直接途径中含P物质的中棘神经元的额外损失导致运动障碍和肌张力障碍 [Galvan et al 2012]。也有证据表明苍白球,丘脑下核,丘脑,下丘脑,黑质和海马神经元丢失[Vonsattel et al 1985, Vonsattel & DiFiglia 1998, Heinsen et al 1999, Petersén et al 2005, Guo et al 2012, Domínguez et al 2013, Singh-Bains et al 2016]。基底神经节和皮层特定区域神经元丢失的可能是受累者最明显的症状,并可能导致个体之间的表型变异[Thu et al 2010, Hadzi et al 2012, Kim et al 2014, Waldvogel et al 2015, Mehrabi et al 2016]。在周围组织中也观察到病理学改变 [Björkqvist et al 2008, van der Burg et al 2009]。
含有亨廷顿蛋白(从HTT表达的蛋白质)的神经内包裹物也是该疾病的重要神经病理学特征。然而,脑中亨廷顿蛋白的表达以及含有亨廷顿蛋白的内含物的模式和时间与疾病的变性无关,并且不被认为是病理的主要决定因素[Kuemmerle et al 1999, Michalik & Van Broeckhoven 2003, Arrasate et al 2004, Slow et al 2005, Slow et al 2006]。
神经影像学。影像学研究为HD的临床诊断提供了额外的支持,并且是研究疾病进展的有价值的工具 [Biglan et al 2009, Paulsen 2009, Tabrizi et al 2011, Tabrizi et al 2012, Tabrizi et al 2013]。除了有症状的个体明显的纹状体萎缩外,还发现了区域和全脑灰白质变化[Majid et al 2011, Tabrizi et al 2011, Tabrizi et al 2012, Tabrizi et al 2013]。此外,MRI研究显示,在疾病发作之前许多年,进行性灰质和白质萎缩[Tabrizi et al 2011, Tabrizi et al 2012, Tabrizi et al 2013]。近年来,许多研究已使用神经影像学来阐明HD的临床进展,特别在临床试验中使用这些客观指标来测试实验性疗法的功效 [Tabrizi et al 2012, Tabrizi et al 2013]。
青少年HD定义为20岁之前的症状发作,占HD患病的5%-10%[Gonzalez-Alegre & Afifi 2006, Quarrell et al 2013]。在成人HD中也观察到了运动,认知和精神障碍,但在青少年HD中也观察到了,但是这些障碍的临床表现是不同的。严重的智力退化,明显的运动和小脑症状,言语和语言延迟以及快速下降也是青少年HD的特征[Nance & Myers 2001, Gonzalez-Alegre & Afifi 2006, Squitieri et al 2006, Yoon et al 2006]。癫痫发作是青少年HD的发作组独特之处,在10岁之前患有HD的发作中占30%-50%[Gonzalez-Alegre & Afifi 2006]。
在青少年中,症状更类似于成人HD,其中舞蹈症和严重的行为障碍是常见的最初表现[Nance & Myers 2001]。
中间等位基因(IA)。不认为CAG重复数在27-35之间的个体有发展为HD的风险,但是由于CAG道的不稳定性,其孩子可能有患病CAG等位基因的风险[Semaka et al 2006, Kay et al 2018]。有限的数据表明,尽管在这方面还需要更多研究,但IAs个体可能表现出行为改变以及运动和认知障碍[Killoran et al 2013, Cubo et al 2016]。
基因型-表型相关
CAG重复次数与HD发病年龄之间存在显著的负相关关系 [Langbehn et al 2004, Langbehn et al 2010]。参见Molecular Genetics。
- 成年症状发作的个体通常具有HTT等位基因CAG的重复范围为36至55。
- 青少年症状发作的个体通常具有HTT等位基因,CAG重复数大于60。
- 中间等位基因(27至35个CAG重复序列)通常不赋予该疾病 表型,但易于发生CAG重复序列不稳定性 [Semaka et al 2013c]。
有关按三核苷酸重复大小发作的特定年龄的可能性的数据,请参阅cmmt.ubc.ca (pdf)。
除了临床发病年龄外,CAG重复长度也可预测死亡年龄,但不能预测疾病的持续时间 [Keum et al 2016]。
随着CAG重复次数的增加,运动,认知和功能指标的恶化率也会增加[Aziz et al 2009, Chao et al 2017]。
行为症状的进展似乎与重复大小无关[Ravina et al 2008]。
完全外显的HD等位基因的纯合子似乎具有与杂合子相似的发病年龄,但可能疾病的发展速度加快[Squitieri et al 2011, Lee et al 2012]。
CAG大小与发作变异性之间也存在显著的负相关性,其中发作后期的变异性较大与较小的CAG相关,这表明非CAG修饰剂对较小的CAG可能比较大的CAG产生更大的影响 [Langbehn et al 2004, Gusella & Macdonald 2009]。平均而言,CAG重复序列大小占发病年龄变异的70%,其中约10%-20%的变异性是由遗传因素引起的 [Li et al 2006, Gusella & Macdonald 2009] 。研究表明,其他基因座上的许多基因占了这种可遗传部分的少量变化[Lee et al 2015]。
近年来,在HTT位点和整个基因组中,在鉴定这些额外的 基因组的修饰方面已取得了重大进展。 HTT启动子中NF-kB转录因子结合位点的变异与基因表达降低和HD发病年龄的改变有关 [Bečanović et al 2015]。当该启动子变体存在于正常的HTT等位基因上时,它与HD发病的早期年龄有关,而当启动子变体存在于引起HD的等位基因上时,观察到相反的作用。此外,全基因组关联研究已开始阐明HD的其他候选修饰基因[Lee et al 2015, Moss et al 2017]。这些分析显示了与该表型有关的生物途径在DNA修复,线粒体裂变和氧化还原酶活性中的重要作用,并确定了候选修饰基因以供将来研究(例如FAN1,MLH1,MTMR10,MSH3和RRM2B )。
外显率
包含36个或更多CAG重复序列的等位基因被认为是导致HD的等位基因,并具有患病的风险。
但是,包含36到39个CAG重复序列的等位基因是不完全外显的,可能导致HD,也可能不会导致HD。具有此范围内CAG重复的老年人无症状者是常见的[Kay et al 2016]。
包含40个以上CAG重复序列的等位基因完全外显。尚无等位基因重复超过40个CAG的无症状老年人的报道。
预后
预后 在HD中会出现疾病的严重性与发病年龄早相关的现象。在突变的等位基因的父系遗传中, 发生得更为普遍。遗传早现的现象是由精子发生过程中CAG重复序列的不稳定性引起的[Semaka et al 2013b]。大的扩增(即等位基因大小增加> 7个CAG重复)几乎仅通过父本遗传发生。患有少年型的儿童通常会从父亲那里继承扩展的等位基因,尽管有时他们会从母亲那里继承[Nahhas et al 2005]。
命名法
在前分子遗传时代,舞蹈病有许多不同的名称,包括圣维特舞蹈团和西登纳姆舞蹈病。
少年HD,或儿童期HD,以前被称为高清的Westphal变异。
尚未出现症状的个体处于HD的预示阶段。被诊断患有舞蹈症和/或其他经过验证的疾病迹象的个体表现为HD。
患病率
全球各地的HD患病率差异很大。欧洲血统的人口平均患病率为每十万分之一9.71 [Rawlins et al 2016],但据报道估计高达每十万分之17 [Fisher & Hayden 2014, Baig et al 2016]。相反,在日本,中国,韩国和芬兰,以及在南非的非洲土著人口中,HD的出现频率要低得多,估计患病率范围为每十万人0.1至2[Pringsheim et al 2012, Sipilä et al 2015, Xu & Wu 2015]。
据信,生活在委内瑞拉马拉开波湖地区的人们是世界上HD病的最高流行地区[Wexler et al 2004]。
HD的不均匀分布至少部分地由这些种族的普通人群中特定的易感等位基因和单倍型的分布来解释[Warby et al 2009, Warby et al 2011, Kay et al 2018]。例如,最近对全球15个不同人群的研究表明,人群和中间 等位基因频率的平均CAG大小与HD患病率相关,并导致主要祖先群体之间疾病患病率的差异[Kay et al 2018]。
HTT等位基因外显率降低(请参见Establishing the Diagnosis,等位基因大小)最近在普通人群中以高频率发生 ,但携带这些等位基因的个体多达400:1 ,尽管低于先前报道的(CAG重复36-38的等位基因为0.2-2%)[Kay et al 2016]。
遗传相关(等位基因)疾病
据报道,HTT中双等位基因致病的错义变异会引起常染色体隐性遗传神经发育障碍Lopes-Maciel-Rodan综合征(LOMARS; OMIM 617435)。 患有LOMARS的个体在儿童时期出现Rett综合征样表型和智力障碍。
鉴别诊断
亨廷顿病(HD)需与舞蹈症,痴呆症和精神疾病鉴别诊断。 几种HD样疾病的鉴别诊断总结于此,并在其他地方进行了综述[Schneider et al 2007, Martino et al 2013]。 也已经报道了阿尔茨海默氏病和HD同时存在 [Davis et al 2014]。
遗传性病因的疾病与舞蹈症需鉴别,但根据相关发现和病程,可疑HD患者很容易被排除。 舞蹈病的病因包括迟发性运动障碍,左旋多巴诱发的运动障碍,甲状腺毒症,脑血管疾病,脑狼疮,红细胞增多症和A组溶血性链球菌。
遗传性病因见 Table 4.
Table 4.
亨廷顿病鉴别诊断中要考虑的遗传疾病
| 疾病 | 基因(s) | MOI | 疾病的临床特征 | |
|---|---|---|---|---|
| 共同点w/HD | 与HD区分 | |||
| Frontotemporal dementia &/or amyotrophic lateral sclerosis( 家族性额颞叶痴呆&/或家族性肌萎缩侧索硬化症) | C9orf72 | AD |
|
|
Huntington disease-like 1 | PRNP | AD | 临床特征相似w/HD |
|
| Huntington disease-like 2 (HDL2)(亨廷顿病样综合征 2) | JPH3 | AD | 在临床上无法与HD区分开 | 在非洲人后裔中患病率最高 & 也许是非洲人后裔独有的 |
| Chorea-acanthocytosis (ChAc)(舞蹈-棘皮症) | VPS13A | AR |
|
|
McLeod neuroacanthocytosis syndrome (MLS) (McLeod神经棘红细胞增多症) | XK | XL |
|
|
Spinocerebellar ataxia type 17 (SCA17) (脊髓小脑共济失调17型) | TBP | AD |
| 小脑共济失调是突出的运动障碍 |
| Dentatorubral-pallidoluysian atrophy (DRPLA)(齿状核红核苍白球路易体萎缩症) | ATN1 | AD |
| 共济失调&肌阵挛是突出的运动障碍 |
| Benign hereditary chorea (OMIM 118700)(良性遗传性舞蹈病) | NKX2-1 | AD | 舞蹈症 |
|
| Hereditary cerebellar ataxia (请参阅Hereditary Ataxia Overview)(遗传性小脑共济失调) | Many | AD AR XL | 运动障碍 | 遗传性小脑共济失调伴有明显的小脑&步态不稳征象 |
Familial Creutzfeld-Jakob disease (家族性克雅氏病) | PRNP | AD |
|
|
Early-onset familial Alzheimer disease (早发家族性阿尔茨海默病) | APP PSEN1 PSEN2 | AD | 痴呆 | 没有运动障碍 |
| Familial frontotemporal dementia with parkinsonism-17(家族性额颞叶痴呆伴帕金森病17) | MAPT | AD |
| 无舞蹈症 |
- 1.
HDL1是由在20p染色体上基因PRNP的特定 致病性变异(8个额外的肽重复)病毒蛋白引起的([Laplanche et al 1999, Moore et al 2001])。 在该位点的相似致病变异还导致其他形式的病毒疾病,例如家族性Creutzfeldt-Jakob疾病(请参阅Genetic Prion Diseases)。
在有HD病史的家庭中,儿童HD病的诊断非常简单。 在单发的病例中(没有已知的HD家族史的受累的),ataxia-telangiectasia, pantothenate kinase-associated neurodegeneration(以前称为Hallervorden-Spatz综合征),Lesch-Nyhan syndrome, Wilson disease,进行性肌阵挛性癫痫[ Gambardella et al 2001]和其他代谢性疾病必须排除在外
处理
初步诊断后的评估
为了确定诊断为亨廷顿病(HD)的个体的疾病程度和需求,建议进行本节概述的评估(如果诊断过程未进行的):
- 体检
- 神经学评估
- 评估与HD相关的全部运动,认知和精神症状。在已描述的一系列临床评分系统中,统一的亨廷顿舞蹈病评分量表(HDRS)提供了对HD的临床特征和进展的可靠且一致的评估。
- 咨询临床遗传学家和/或遗传咨询师
表现治疗
药物治疗仅限于对症治疗 [Mestre et al 2009, Killoran & Biglan 2014]。
- 一般(氟哌啶醇)和非典型(奥氮平)抗精神病药可部分抑制舞蹈运动。苯二氮卓类;或多巴胺耗竭剂丁苯那嗪[de Tommaso et al 2005, Bonelli & Wenning 2006, Huntington Study Group 2006]。丁苯那嗪可以有效地作为一种抗胆碱药。但是,其使用会带来严重的不良反应,例如锥体束外症状。丁苯那嗪的类似物Deutetrabenazine已经通过分子上特定位置的氘原子取代进行了修饰,以增加半衰期和暴露 [Stamler et al 2013]。这些特性使得给药频率降低,不良反应更少[Huntington Study Group 2016, Reilmann 2016]。
- 抗帕金森病药物可改善运动功能减退和僵硬,但可能增加舞蹈症。
- 精神症状如抑郁症,精神病性症状和伤人通常对精神药物或某些类型的抗癫痫药反应良好。
- 丙戊酸改善了亨廷顿病的肌阵挛性运动亢进[Saft et al 2006]。
患有HD的人及其家人非常需要支持护理,包括护理,饮食,专用设备以及获得州和联邦福利。许多社会问题困扰着HD及其家人。实际的帮助,情感支持和咨询可以减轻压力[Williams et al 2009]。
预防继发并发症
HD的主要继发并发症包括:
- 任何需要长期支持治疗的个体通常观察到的并发症
- 与各种药物治疗有关的副作用。药物的副作用取决于多种因素,包括所涉及的化合物,剂量和个体。但是使用通常用于HD的药物,副作用可能包括抑郁,镇静,恶心,躁动不安,头痛,中性粒细胞减少和迟发性运动障碍。对于某些人来说,某些治疗方法的副作用可能比症状更严重。这样的个体将从治疗中移出,减少剂量或定期从治疗中“休息”。当前用于治疗舞蹈病的药物特别容易产生明显的副作用。轻度至中度舞蹈病患者可以通过运动训练和言语治疗等非药物疗法得到更好的帮助。
- 抑郁。当有指征时,标准治疗是合适的[Paulsen et al 2005b, Phillips et al 2008]。
监测
应该进行定期评估,以解决舞蹈症的外观和严重程度,僵硬,步态问题,抑郁,行为改变和认知能力下降[Anderson & Marshall 2005, Skirton 2005]。
行为观察量表亨廷顿行为量表(BOSH)是为在养老院环境中快速和纵向评估HD患者的功能能力而开发的量表[Timman et al 2005]。 对于纵向研究,使用统一高清评分量表(UHDRS)[Huntington Study Group 1996, Siesling et al 1998, Youssov et al 2013]。 总功能量(TFC)量表用于描述HD的进展,功能水平以及对其他看护者援助的要求(TFC scale)。
避免的药物/情况
含左旋多巴的化合物可能会加重舞蹈症。
不鼓励饮酒和吸烟。
评估处于危险中亲戚
有关与遗传咨询目的有关的高危亲属测试的问题,请参见Genetic Counseling 。
正在调查的疗法
在 HD动物模型和人类临床试验中,各种各样的潜在疗法正在研究中[Wild & Tabrizi 2014]。 这种多样性反映了HD中涉及的多种细胞途径。[Bonelli et al 2004, Rego & de Almeida 2005, Borrell-Pagès et al 2006, Graham et al 2006, Bonelli & Hofmann 2007].
- 正在研究的药物包括以下抑制剂:凋亡,兴奋性毒性,亨廷顿蛋白聚集,亨廷顿蛋白水解,亨廷顿蛋白磷酸化,炎症,氧化损伤,磷酸二酯酶活性,组蛋白脱乙酰酶和转谷氨酰胺酶活性; 以及调节线粒体功能,伴侣的活性,转录和神经营养支持的化合物。
- 在HD的临床前动物模型中显示出改善并已进入临床试验的治疗药物包括米诺环素,丁酸钠,必需脂肪酸,消旋剂,肌酸,胱胺,利鲁唑,美金刚,白藜芦醇,大麻提取物(THC和CBD),辅酶Q10 ,和磷酸二酯酶10a抑制剂[Bender et al 2005, Puri et al 2005, Bonelli & Wenning 2006, Ondo et al 2007, Okamoto et al 2009, Huntington Study Group DOMINO Investigators 2010, Hersch et al 2017, McGarry et al 2017]。 值得注意的是,这些治疗剂中的许多都未能达到临床治疗标准并显示出改善HD临床进展的功效。 包括普利多匹定,拉喹莫德和semaphorin-4D中和抗体在内的实验性治疗药物仍在临床开发中。
- 在临床前动物研究中,针对HD的基因沉默方法已被证明是安全有效的,并且目前正在或即将进入临床试验。这些方法包括使用RNA干扰(RNAi)或反义寡核苷酸(ASO)的方法 [Boudreau et al 2009, Pfister & Zamore 2009, Hu et al 2010, McBride et al 2011, Kordasiewicz et al 2012, Aronin & DiFiglia 2014, Dufour et al 2014, Stanek et al 2014, Pfister et al 2018]。这些方法旨在以非选择性方式沉默所有亨廷顿蛋白的表达 [Boudreau et al 2009, McBride et al 2011, Kordasiewicz et al 2012]或仅对突变的HTT 等位基因具有 选择性[Gagnon et al 2010, Carroll et al 2011, Evers et al 2011, Skotte et al 2014, Southwell et al 2014, Datson et al 2017]。通过靶向扩增的CAG区域 [Gagnon et al 2010, Evers et al 2011, Datson et al 2017]或靶向具有CAG扩增的连锁不平衡中的多态性 [Carroll et al 2011, Skotte et al 2014, Southwell et al 2014, Kay et al 2015]。来自第一项针对HTT靶向ASO的人类I / IIa阶段临床试验的主要数据显示,IONIS-HTTRx(RG6042)在所有测试剂量下均具有良好的耐受性,并导致脑脊液中mHTT的剂量依赖性降低( CSF)(IONIS™ press release 3-1-18)。
- 在 HD中进行细胞移植研究显示出少量个体的可变结果[Furtado et al 2005, Bachoud-Lévi et al 2006, Farrington et al 2006, Dunnett & Rosser 2007, Barker et al 2013]; 然而,更多的大型研究正在进行中[Lopez et al 2014]。 此外,静脉注射间充质干细胞的安全性和功效目前正在HD个体的首次人类临床试验中进行测试(NCT02728115)。 令人关注的是,最近的研究表明突变的亨廷顿蛋白能够扩散到同种异体移植的神经组织中[Cicchetti et al 2014]。
有关HD的已计划或正在进行许多人类临床试验,并在 www.huntington-study-group.org上列出。 许多药物试验已经完成和/或正在进行中。 例如,高剂量肌酸试验和使用SD-809(丁苯那嗪的改良形式[Xenazine®,Nitoman®])的试验都处于III期临床试验中。 PBT2的II期临床试验也在进行中。 有关最新信息,请参见 hddrugworks.org。
搜索美国 ClinicalTrials.gov,搜索欧洲www.ClinicalTrialsRegister.eu,以获取有关各种疾病和状况的临床研究信息。
其他
已经进行了生物标志物研究,例如TRACK-HD,PREDICT-HD和ENROLL-HD,以通过成像,临床量表和生理学测量来识别疾病进展的早期变化[Scahill et al 2012, Ross et al 2014]。还已经对有HD危险的人进行了纵向研究 [Huntington Study Group PHAROS Investigators 2006, Paulsen et al 2006, Tabrizi et al 2012].
在HD患者队列中评估了许多疾病发作和临床进展的候选分子生物标志物,但只有少数经过了验证。研究表明,脑脊液中亨廷顿蛋白的突变水平与HD的疾病阶段有关[Southwell et al 2015, Wild et al 2015],并可能反映脑中mHTT的水平 [Southwell et al 2015]。因此,CSF中的mHTT水平可提供可靠的临床进展生物标志物,并可为以HTT为目标的临床试验提供大脑中HTT抑制的替代指标。此外,血液和脑脊液中神经丝轻链的水平已被证明是患有HD的个体疾病发作和临床进展以及区域性脑萎缩的潜在预后生物标志物 [Byrne et al 2017, Johnson et al 2018]。
与父母一起受累的HD患儿和青少年,有时处于非常贫困的状况,可能会有特殊问题。推荐给当地的HD支持小组以获取教育材料和所需的心理支持会很有帮助(请参阅 Resources)。
Donepezil(一种用于治疗阿尔茨海默氏病的药物)并没有改善HD的运动或认知功能[Cubo et al 2006]。
遗传咨询
遗传咨询是为个人和家庭提供有关遗传疾病的性质,遗传和影响的信息,以帮助他们做出明智的医疗和个人决定的过程。 以下部分介绍了遗传风险评估以及家族史和基因检测的使用,以阐明家族成员的遗传状况。 本部分的目的不是要解决个人可能面临的所有个人,文化或伦理问题,也不能替代遗传专家的咨询。 —编者。
遗传方式
亨廷顿舞蹈病(HD) 为常染色体显性遗传 .
家庭成员的风险
先证者的父母
先证者的同胞。 先证者同胞的风险取决于先证者父母的遗传状况 :
先证者的后代
- 在受孕时,由于HTT中CAG重复扩增的杂合性导致HD个体的每个孩子都有50%的机会遗传HD 等位基因.
- 过渡区等位基因(IA):27-35 CAG。 具有过渡区等位基因的个体的后代有发生HD的风险,因为CAG重复序列在世代之间传递时的不稳定性会导致CAG重复序列的扩增[Semaka et al 2006, Semaka et al 2013b]。 具有中等等位基因的个体的遗传咨询尤其具有挑战性,因为其子女的临床结果尚不确定[Semaka et al 2013a]。 对于孩子而言,从过渡区等位基因的亲代继承CAG扩展大于35个重复序列或“导致新HD的等位基因”的风险取决于多种因素,包括以下因素:
- 等位基因的CAG大小。 较大的CAG更易于扩展。据报道,精子CAG大小重复不稳定估计值可以使遗传咨询师为接受IA预测测试结果的人提供更准确的风险评估 [Hendricks et al 2009, Semaka et al 2013b, Semaka & Hayden 2014]。 虽然显示出所有过渡区CAG重复序列大小都有扩展的可能性,但随着CAG大小的增加,扩展的可能性也会大大增加。 35个CAG等位基因中约有21%扩展到与疾病相关的范围。Semaka & Hayden [2014]已发布了基于证据的 遗传咨询对过渡区等位基因预测测试结果的启示。
- 父母的性别和年龄。 从父亲遗传的过渡区等位基因比母亲遗传的过渡区等位基因更容易发生CAG扩增。 产妇的扩张极为罕见 [Semaka et al 2015]。 扩展的过渡区等位基因优先由父本高龄的男性传播。
- 带有cis构型的DNA序列具有CAG扩展。 被CAA和CCG三核苷酸打断的CAG区域更稳定。
其他家庭成员。 其他家庭成员的风险取决于 先证者'父母的遗传状况:如果父母受累的或有HTT的CAG扩展,则他或她的家庭成员有风险。
相关的遗传咨询问题
预测性测试(即无症状的高风险个体的测试)。 可以对无症状成人进行HD危险性测试。 在没有明确症状的情况下进行致病性变异的检测是预测性检测。 这种测试对准确预测无症状个体的发病年龄,严重程度,症状类型或进展速度没有帮助。 然而, Langbehn et al [2004]和Langbehn et al [2010]报道的有关具有特定CAG重复大小的个体在特定年龄受累的可能性的数据可能是有用的。 请参阅cmmt.ubc.ca (pdf)上的补充表。 在对高危人群进行HD检测时,测试受影响家庭成员的HTT中CAG扩展是有用的,以确认该家庭的疾病是HD。
- 有风险且无症状的成年家庭成员可能会寻求测试,以便就生殖,财务和职业规划做出个人决定。有风险的无症状个体也可能有资格参加临床试验。其他人可能有不同的动机,包括简单的“需要知道”。无症状高危成年家庭成员的测试通常包括测试前访谈,其中要求进行测试的动机,个人对HD的了解,测试结果阳性和阴性的可能影响以及神经系统状态的评估。应为那些寻求测试的人提供咨询,告知他们可能遇到的有关健康,生命和残疾保险,就业和教育歧视以及社会和家庭互动变化的问题。有趣的是,一项研究发现,基因检测不会增加歧视的风险。可能是由于HD的家族史而不考虑基因状态,而不是由于HD基因测试的特定结果,所以人们可能会感觉到遗传歧视[Bombard et al 2009]。其他需要考虑的问题包括对其他家庭成员处于危险状态的影响[Bombard et al 2012]。抑郁和自杀观念是HD预测测试程序中需要解决的问题 [Robins Wahlin et al 2000, Robins Wahlin 2007]。应征得知情同意,并对记录保密。等位基因发生突变的个体需要安排长期随访和评估。
- 对加拿大预测性测试计划参与者的短期随访表明,对HD的预测性测试可以维持甚至改善处于危险中的人的心理健康,即使其中一些人的经历是负面的。 被确定处于降低风险中的该组中,约有10%的人在适应新状况方面存在严重困难。 这些人的主要问题是意识到他们正面临计划外的未来。 总体而言,对有风险的无症状成年人进行测试的需求已经比在直接分子遗传学检测可用之前进行的研究中的预期要低。 一般而言,与医疗服务和基因检测的使用相一致,女性比男性更有可能接受 HD预测性检测[Taylor 2005, Baig et al 2016]。
- Decruyenaere et al [2005] 患有HD 等位基因的无症状个体的伴侣的心理困扰研究中发现, 伴侣的困扰相同。 他们往往被“剥夺了公民权”或未被社会认可而的悲伤。
对未成年人的预测性测试(即,对18岁以下无症状高危个体的测试)
- 对于无症状成年发病的未成年人, 对其进行早期治疗不会对疾病的发病率和死亡率产生有益的影响,因此认为预测性基因检测是不合适的,主要是因为它否定了儿童的自主权,并没有令人信服的好处。 此外,人们担心此类信息可能会对家庭动态产生潜在的不健康不良影响,将来受到歧视和污名化的风险以及此类信息可能引起的焦虑。
- 欲了解更多信息,请参阅美国国家遗传咨询师协会关于未成年人成年发作情况的基因检测的 position statement,以及美国儿科学会和美国医学遗传与基因组学院的policy statement:基因检测和筛查中的伦理和政策问题 孩子们。
- 在确诊为HD的家庭中,考虑对有症状个体进行测试是合适的,而不论其年龄如何。I
具有明显de novo 致病性变异的家庭的注意事项。 当父母双方都不具有导致高清的HTT等位基因(> 35个CAG重复序列)或过渡区等位基因(27-35个重复序列)时,考虑非医学解释,包括非生物学父亲或产妇(例如,辅助生殖)和未公开收养。
家庭计划
- 确定遗传风险和讨论产前检查可用性的最佳时间是在怀孕之前。
- 同样,最好在怀孕之前做出有关确定无症状高危家庭成员遗传状况的测试决定。
DNA库是DNA(通常从白细胞中提取)的存储,以备将来使用。因为将来测试方法和我们对基因,等位基因变异和疾病的理解可能会有所改善,所以应考虑受累的银行DNA。
产前检查和植入前遗传学诊断
对于胎儿,风险为50%。如果已经在 受累的父母或高危父母的患病亲戚中确认了导致HD的HTT等位基因的存在,则可以对风险增加的妊娠进行产前检查。
对于已经在 受累的家庭成员中鉴定出引起HD的HTT等位基因的家庭,植入前遗传学诊断(PGD)可能是一种选择。现有的PGD排除方案允许对高危家庭中不希望自己进行症状前测试的夫妇进行胚胎测试[Sermon et al 2002, Stern et al 2002, Moutou et al 2004, Jasper et al 2006]。 Asscher & Koops [2010]讨论了影响HDPGD的咨询和道德问题。
当考虑出于终止妊娠或早期诊断的目的而考虑进行产前检查时,医学专业人员和家庭内部可能存在观点差异。尽管大多数中心将有关产前检查的决定视为父母的选择,但对这些问题的讨论是适当的。
资源
GeneReviews工作人员选择了以下特定疾病和/或综合保护组织和/或注册表,以保护患有这种疾病的个人及其家人。 GeneReviews对其他组织提供的信息概不负责。 有关选择标准的信息,请单 here.
- European Huntington's Disease Network (EHDN)Germany
- HDBuzz
- Huntington Society of Canada151 Frederick StreetSuite 400Kitchener Ontario N2H 2M2CanadaPhone: 800-998-7398 (toll-free); 519-749-7063Fax: 519-749-8965Email: info@huntingtonsociety.ca
- Huntington’s Disease AfricaPhone: 254746734559Email: info@hd-africa.org
- Huntingtons Association of South Africa (HASA)South Africa
- Huntington's Disease Society of America (HDSA)505 Eighth AvenueSuite 902New York NY 10018Phone: 800-345-4372 (toll-free); 212-242-1968Fax: 212-239-3430Email: hdsainfo@hdsa.org
- International Huntington AssociationNetherlandsEmail: svein@iha-huntington.org
- La Société Huntington du Québec (Huntington Society of Quebec)Montréal QuebecCanadaPhone: 514-282-4272; 877-282-2444; 877-220-0226Fax: 514-937-0082Email: shq@huntingtonqc.org
- National Library of Medicine Genetics Home Reference
- Testing for Huntington Disease: Making an Informed ChoiceBooklet providing information about Huntington disease and genetic testingUniversity of Washington Medical CenterSeattle WA
- Hereditary Disease Foundation3960 Broadway6th FloorNew York NY 10032Phone: 212-928-2121Fax: 212-928-2172Email: cures@hdfoundation.org
- European Huntington's Disease Network (EHDN) RegistryGermany
- Huntington Study Group (HSG)University of Rochester, HSG Administrative Office1351 Mount Hope AvenueSuite 223Rochester NY 14620Phone: 800-487-7671 (toll-free)
分子遗传
分子遗传学和OMIM表中的信息可能与GeneReview中其他地方的信息不同:表可能包含最新信息。 —编者.
亨廷顿病的OMIM条目 (View All in OMIM)
基因结构 HTT(NCBI基因ID:3064)涵盖67个外显子,跨度近170 kb。 HTT主要表达为13.5kb的转录本(NM_002111.8)。据报道,某些患者出现了较短的转录本,这是由于3'UTR的多聚腺苷酸化导致的。尽管这些转录本不存在于转录本数据库中 [Lin et al 1993, Romo et al 2017]。该基因包含一个 三核苷酸重复(CAG),该核苷酸在HTT内扩展到至少一个患有亨廷顿病(HD)的个体的 染色体上。有关基因和蛋白质信息的详细摘要,请参见 Table A,基因。
良性变异。在人群中,CAG重复序列的长度高度多态,未受影响的等位基因的CAG重复序列的大小范围为9到35。等位基因 的中值大小为18个CAG。在所有人群中,最常见的等位基因包含15-20个CAG的重复序列[Warby et al 2009]。中间等位基因的CAG重复序列范围为27至35 [Semaka et al 2013b]。
致病变异。 HD的致病性变异是HTT的第一个外显子中CAG三核苷酸(或聚谷氨酰胺)束的扩展。 HD患者的CAG重复长度为36或更大。成人HD发作的个体CAG扩展通常从40增加到55,而青少年发病个体的CAG扩展通常大于60,通常是由父亲遗传的。在CAG重复长度和发病年龄之间存在公认的反相关关系。但是,CAG重复范围为36-39的等位基因的外显率降低。
Table 5.
Table 5
本GeneReview中讨论的HTT变异
| 变异分类 | DNA核苷酸改变 | 蛋白质改变 | 参考序列 |
|---|---|---|---|
| 良性 | c.52_54CAG[9_26] (≤26 CAG repeats) | p.Gln18[9_26] | NM_002111 NP_002102 |
| c.52CAG[27_35] 2 (27 to 35 CAG repeats) | p.Gln18[27_35] | ||
| 致病性 | c.52CAG[36_39] 3 (36 to 39 CAG repeats) | p.Gln18[36_39] | |
| c.52CAG[40_?] 4 (≥40 CAG repeats) | p.Gln18[40_?] |
有关变异分类的注意事项:表中列出的变异由作者提供。 GeneReviews员工尚未独立验证变体的分类。
关于术语的注释:GeneReviews遵循人类基因组变异学会 (varnomen- .hgvs.org )的标准命名约定。 有关命名法的说明,请参见Quick Reference。- 1.
对于参考蛋白质序列,NP_002102
- .4 具有3,144个氨基酸。 第18位的第一个Gln残基存在23份; 它被指定为p.Gln18 [23]。- 2.
中间HTT等位基因
- 3.
HD的HTT等位基因的外显率降低
- 4.
引起HD的全外显率HTT等位基因
正常基因产物。 亨廷顿蛋白是一种具有3,144个氨基酸的蛋白质 (NP_002102.4),最近已使用冷冻电子显微镜对348 kd HTT蛋白的结构进行了解析 [Guo et al 2018]。 亨廷顿蛋白被广泛表达,而突变和野生型蛋白的区域分布没有明显差异。 聚谷氨酰胺束从残基18开始,然后是聚脯氨酸区域。 聚谷氨酰胺束下游的区域含有一个HEAT重复序列,该序列由40个松散保守的氨基酸串联而成,并重复多次重复,被认为与蛋白质-蛋白质相互作用有关[Palidwor et al 2009]。
异常基因产物 。 HTT中的CAG重复序列被翻译成不间断的谷氨酰胺残基序列,当扩展时可能会产生结构和生化特性的产物。
参考文献
发布的准则/共识声明
- American College of Medical Genetics/American Society of Human Genetics Huntington Disease Genetic Testing Working Group. Laboratory guidelines for Huntington disease genetic testing. Am J Hum Genet. 1998;62:1243 - 7. [PMC free article: PMC1377103] [PubMed: 9545416]
- Committee on Bioethics, Committee on Genetics, and American College of Medical Genetics and Genomics Social, Ethical, Legal Issues Committee. Ethical and policy issues in genetic testing and screening of children. Available online. 2013. Accessed 10-18-18. [PubMed: 23428972]
- International Huntington Association and the World Federation of Neurology Research Group on Huntington's Chorea. Guidelines for the molecular genetics predictive test in Huntington's disease. Neurology. 1994;44:1533 - 6. [PubMed: 8058167]
- International Huntington Association and World Federation of Neurology. Guidelines for the molecular genetic predictive test in Huntington's disease. Available online. 1994. Accessed 10-18-18.
- National Society of Genetic Counselors. Position statement on genetic testing of minors for adult-onset conditions. Available online. 2017. Accessed 10-18-18.
- World Federation of Neurology Research Committee Research Group on Huntington's Chorea. Ethical issues policy statement on Huntington's chorea molecular genetics predictive test. J Neurol Sci. 1989;94:327 - 32. [PubMed: 2533250]
Literature Cited
- Anderson KE, Marshall FJ. Behavioral symptoms associated with Huntington's disease. Adv Neurol. 2005;96:197 - 208. [PubMed: 16383221]
- Aronin N, DiFiglia M. Huntingtin-lowering strategies in Huntington's disease: antisense oligonucleotides, small RNAs, and gene editing. Mov Disord. 2014;29:1455 - 61. [PubMed: 25164989]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805 - 10. [PubMed: 15483602]
- Asscher E, Koops B-J. The right not to know and preimplantation genetic diagnosis for Huntington's disease. J Med Ethics. 2010;36:30 - 3. [PubMed: 20026690]
- Aziz NA, Jurgens CK, Landwehrmeyer GB, et al. Normal and mutant HTT interact to affect clinical severity and progression in Huntington disease. Neurology. 2009;73:1280 - 5. [PubMed: 19776381]
- Bachoud-Lévi AC, Gaura V, Brugieres P, Lefaucheur JP, Boisse MF, Maison P, Baudic S, Ribeiro MJ, Bourdet C, Remy P, Cesaro P, Hantraye P, Peschanski M. Effect of fetal neural transplants in patients with Huntington's disease 6 years after surgery: a long-term follow-up study. Lancet Neurol. 2006;5:303 - 9. [PubMed: 16545746]
- Baig SS, Strong M, Rosser E, Taverner NV, Glew R, Miedzybrodzka Z, Clarke A, Craufurd D., UK Huntington's Disease Prediction Consortium. Quarrell OW. 22 years of predictive testing for Huntington's disease: the experience of the UK Huntington's Prediction Consortium. Eur J Hum Genet. 2016;24:1396 - 402. [PMC free article: PMC5027682] [PubMed: 27165004]
- Baliko L, Csala B, Czopf J. Suicide in Hungarian Huntington's disease patients. Neuroepidemiology. 2004;23:258 - 60. [PubMed: 15316254]
- Barker RA, Mason SL, Harrower TP, Swain RA, Ho AK, Sahakian BJ, Mathur R, Elneil S, Thornton S, Hurrelbrink C, Armstrong RJ, Tyers P, Smith E, Carpenter A, Piccini P, Tai YF, Brooks DJ, Pavese N, Watts C, Pickard JD, Rosser AE, Dunnett SB, et al. The long-term safety and efficacy of bilateral transplantation of human fetal striatal tissue in patients with mild to moderate Huntington's disease. J Neurol Neurosurg Psychiatry. 2013;84:657 - 65. [PMC free article: PMC3646287] [PubMed: 23345280]
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ. Huntington disease. Nat Rev Dis Primers. 2015;1:15005. [PubMed: 27188817]
- Bean L, Bayrak-Toydemir P. American College of Medical Genetics and Genomics Standards and Guidelines for Clinical Genetics Laboratories, 2014 edition: technical standards and guidelines for Huntington disease. Genet Med. 2014;16:e2. [PubMed: 25356969]
- Bečanović K, Nørremølle A, Neal SJ, Kay C, Collins JA, Arenillas D, Lilja T, Gaudenzi G, Manoharan S, Doty CN, Beck J, Lahiri N, Portales-Casamar E, Warby SC, Connolly C, De Souza RA. REGISTRY Investigators of the European Huntington's Disease Network, Tabrizi SJ, Hermanson O, Langbehn DR, Hayden MR, Wasserman WW, Leavitt BR. A SNP in the HTT promoter alters NF-κB binding and is a bidirectional genetic modifier of Huntington disease. Nat Neurosci. 2015;18:807 - 16. [PubMed: 25938884]
- Bender A, Auer DP, Merl T, Reilmann R, Saemann P, Yassouridis A, Bender J, Weindl A, Dose M, Gasser T, Klopstock T. Creatine supplementation lowers brain glutamate levels in Huntington's disease. J Neurol. 2005;252:36 - 41. [PubMed: 15672208]
- Benjamin CM, Adam S, Wiggins S, Theilmann JL, Copley TT, Bloch M, Squitieri F, McKellin W, Cox S, Brown SA, et al. Proceed with care: direct predictive testing for Huntington disease. Am J Hum Genet. 1994;55:606 - 17. [PMC free article: PMC1918294] [PubMed: 7942838]
- Biglan KM, Ross CA, Langbehn DR, Aylward EH, Stout JC, Queller S, Carlozzi NE, Duff K, Beglinger LJ, Paulsen JS, et al. Motor abnormalities in premanifest persons with Huntington's disease: the PREDICT-HD study. Mov Disord. 2009;24:1763 - 72. [PMC free article: PMC3048804] [PubMed: 19562761]
- Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, Leavitt BR, Möller T, Tabrizi SJ. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J Exp Med. 2008;205:1869 - 77. [PMC free article: PMC2525598] [PubMed: 18625748]
- Blekher T, Johnson SA, Marshall J, White K, Hui S, Weaver M, Gray J, Yee R, Stout JC, Beristain X, Wojcieszek J, Foroud T. Saccades in presymptomatic and early stages of Huntington disease. Neurology. 2006;67:394 - 9. [PubMed: 16855205]
- Bombard Y, Palin J, Friedman JM, Veenstra G, Creighton S, Bottorff JL, Hayden MR, et al. Beyond the patient: the broader impact of genetic discrimination among individuals at risk of Huntington disease. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:217 - 26. [PubMed: 22231990]
- Bombard Y, Veenstra G, Friedman JM, Creighton S, Currie L, Paulsen JS, Bottorff JL, Hayden MR, et al. Perceptions of genetic discrimination among people at risk for Huntington's disease: a cross sectional survey. BMJ. 2009;338:b2175. [PMC free article: PMC2694258] [PubMed: 19509425]
- Bonelli RM, Hofmann P. A systematic review of the treatment studies in Huntington's disease since 1990. Expert Opin Pharmacother. 2007;8:141 - 53. [PubMed: 17257085]
- Bonelli RM, Wenning GK. Pharmacological management of Huntington's disease: an evidence-based review. Curr Pharm Des. 2006;12:2701 - 20. [PubMed: 16842168]
- Bonelli RM, Wenning GK, Kapfhammer HP. Huntington's disease: present treatments and future therapeutic modalities. Int Clin Psychopharmacol. 2004;19:51 - 62. [PubMed: 15076012]
- Borrell-Pagès M, Zala D, Humbert S, Saudou F. Huntington's disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci. 2006;63:2642 - 60. [PubMed: 17041811]
- Boudreau RL, McBride JL, Martins I, Shen S, Xing Y, Carter BJ, Davidson BL. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington's disease mice. Mol Ther. 2009;17:1053 - 63. [PMC free article: PMC2835182] [PubMed: 19240687]
- Bourne C, Clayton C, Murch A, Grant J. Cognitive impairment and behavioural difficulties in patients with Huntington's disease. Nurs Stand. 2006;20:41 - 4. [PubMed: 16722121]
- Byrne LM, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RAC, Scahill RI, Tabrizi SJ, Zetterberg H, Langbehn D, Wild EJ. Neurofilament light protein in blood as a potential biomarker of neurodegeneration in Huntington's disease: a retrospective cohort analysis. Lancet Neurol. 2017;16:601 - 9. [PMC free article: PMC5507767] [PubMed: 28601473]
- Carroll JB, Warby SC, Southwell AL, Doty CN, Greenlee S, Skotte N, Hung G, Bennett CF, Freier SM, Hayden MR. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene / allele-specific silencing of mutant huntingtin. Mol Ther. 2011;19:2178 - 85. [PMC free article: PMC3242664] [PubMed: 21971427]
- Chao TK, Hu J, Pringsheim T. Risk factors for the onset and progression of Huntington disease. Neurotoxicology. 2017;61:79 - 99. [PubMed: 28111121]
- Cicchetti F, Lacroix S, Cisbani G, Vallières N, Saint-Pierre M, St-Amour I, Tolouei R, Skepper JN, Hauser RA, Mantovani D, Barker RA, Freeman TB. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann Neurol. 2014;76:31 - 42. [PubMed: 24798518]
- Cubo E, Ramos-Arroyo MA, Martinez-Horta S, Martínez-Descalls A, Calvo S, Gil-Polo C, European HD. Network. Clinical manifestations of intermediate allele carriers in Huntington disease. Neurology. 2016;87:571 - 8. [PubMed: 27402890]
- Cubo E, Shannon KM, Tracy D, Jaglin JA, Bernard BA, Wuu J, Leurgans SE. Effect of donepezil on motor and cognitive function in Huntington disease. Neurology. 2006;67:1268 - 71. [PubMed: 17030764]
- Datson NA, González-Barriga A, Kourkouta E, Weij R, van de Giessen J, Mulders S, Kontkanen O, Heikkinen T, Lehtimäki K, van Deutekom JC. The expanded CAG repeat in the huntingtin gene as target for therapeutic RNA modulation throughout the HD mouse brain. PLoS One. 2017;12:e0171127. [PMC free article: PMC5300196] [PubMed: 28182673]
- Davis MY, Keene CD, Jayadev S, Bird T. The co-occurrence of Alzheimer's disease and Huntington's disease: a neuropathological study of 15 elderly Huntington's disease subjects. J Huntingtons Dis. 2014;3:209 - 17. [PubMed: 25062863]
- de Tommaso M, Di Fruscolo O, Sciruicchio V, Specchio N, Cormio C, De Caro MF, Livrea P. Efficacy of levetiracetam in Huntington disease. Clin Neuropharmacol. 2005;28:280 - 4. [PubMed: 16340384]
- Decruyenaere M, Evers-Kiebooms G, Boogaerts A, Demyttenaere K, Dom R, Fryns JP. Partners of mutation-carriers for Huntington's disease: forgotten persons? Eur J Hum Genet. 2005;13:1077 - 85. [PubMed: 15999117]
- Djoussé L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington's disease. Neurology. 2002;59:1325 - 30. [PubMed: 12427878]
- Domínguez D JF, Egan GF, Gray MA, Poudel GR, Churchyard A, Chua P, Stout JC, Georgiou-Karistianis N. Multi-modal neuroimaging in premanifest and early Huntington's disease: 18 month longitudinal data from the IMAGE-HD study. PLoS One. 2013;8:e74131. [PMC free article: PMC3774648] [PubMed: 24066104]
- Duan W, Jiang M, Jin J. Metabolism in HD: still a relevant mechanism? Mov Disord. 2014;29:1366 - 74. [PMC free article: PMC4163071] [PubMed: 25124273]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC, et al. Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biol Psychiatry. 2007;62:1341 - 6. [PubMed: 17481592]
- Dufour BD, Smith CA, Clark RL, Walker TR, McBride JL. Intrajugular vein delivery of AAV9-RNAi prevents neuropathological changes and weight loss in Huntington's disease mice. Mol Ther. 2014;22:797 - 810. [PMC free article: PMC3982495] [PubMed: 24390280]
- Dunnett SB, Rosser AE. Stem cell transplantation for Huntington's disease. Exp Neurol. 2007;203:279 - 92. [PubMed: 17208230]
- Eddy CM, Parkinson EG, Rickards HE. Changes in mental state and behaviour in Huntington's disease. Lancet Psychiatry. 2016;3:1079 - 86. [PubMed: 27663851]
- Epping EA, Kim JI, Craufurd D, Brashers-Krug TM, Anderson KE, McCusker E, Luther J, Long JD, Paulsen JS., PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Longitudinal psychiatric symptoms in prodromal Huntington's disease: a decade of data. Am J Psychiatry. 2016;173:184 - 92. [PMC free article: PMC5465431] [PubMed: 26472629]
- Evers MM, Pepers BA, van Deutekom JC, Mulders SA, den Dunnen JT, Aartsma-Rus A, van Ommen GJ, van Roon-Mom WM. Targeting several CAG expansion diseases by a single antisense oligonucleotide. PLoS One. 2011;6:e24308. [PMC free article: PMC3164722] [PubMed: 21909428]
- Farrington M, Wreghitt TG, Lever AM, Dunnett SB, Rosser AE, Barker RA. Neural transplantation in Huntington's disease: the NEST-UK donor tissue microbiological screening program and review of the literature. Cell Transplant. 2006;15:279 - 94. [PubMed: 16898222]
- Fisher ER, Hayden MR. Multisource ascertainment of Huntington disease in Canada: prevalence and population at risk. Mov Disord. 2014;29:105 - 14. [PubMed: 24151181]
- Furtado S, Sossi V, Hauser RA, Samii A, Schulzer M, Murphy CB, Freeman TB, Stoessl AJ. Positron emission tomography after fetal transplantation in Huntington's disease. Ann Neurol. 2005;58:331 - 7. [PubMed: 16049929]
- Gaba AM, Zhang K, Marder K, Moskowitz CB, Werner P, Boozer CN. Energy balance in early-stage Huntington disease. Am J Clin Nutr. 2005;81:1335 - 41. [PubMed: 15941884]
- Gagnon KT, Pendergraff HM, Deleavey GF, Swayze EE, Potier P, Randolph J, Roesch EB, Chattopadhyaya J, Damha MJ, Bennett CF, Montaillier C, Lemaitre M, Corey DR. Allele-selective inhibition of mutant huntingtin expression with antisense oligonucleotides targeting the expanded CAG repeat. Biochemistry. 2010;49:10166 - 78. [PMC free article: PMC2991413] [PubMed: 21028906]
- Galvan L, André VM, Wang EA, Cepeda C, Levine MS. Functional differences between direct and indirect striatal output pathways in Huntington's disease. J Huntingtons Dis. 2012;1:17 - 25. [PMC free article: PMC4116084] [PubMed: 25063187]
- Gambardella A, Muglia M, Labate A, Magariello A, Gabriele AL, Mazzei R, Pirritano D, Conforti FL, Patitucci A, Valentino P, Zappia M, Quattrone A. Juvenile Huntington's disease presenting as progressive myoclonic epilepsy. Neurology. 2001;57:708 - 11. [PubMed: 11524486]
- Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology. 2006;67:485 - 7. [PubMed: 16625001]
- Gonzalez-Alegre P, Afifi AK. Clinical characteristics of childhood-onset (juvenile) Huntington disease: report of 12 patients and review of the literature. J Child Neurol. 2006;21:223 - 9. [PubMed: 16901424]
- Goodman AO, Barker RA. How vital is sleep in Huntington's disease? J Neurol. 2010;257:882 - 97. [PubMed: 20333394]
- Graham RK, Slow EJ, Deng Y, Bissada N, Lu G, Pearson J, Shehadeh J, Leavitt BR, Raymond LA, Hayden MR. Levels of mutant huntingtin influence the phenotypic severity of Huntington disease in YAC128 mouse models. Neurobiol Dis. 2006;21:444 - 55. [PubMed: 16230019]
- Guo Q, Huang Bin, Cheng J, Seefelder M, Engler T, Pfeifer G, Oeckl P, Otto M, Moser F, Maurer M, Pautsch A, Baumeister W, Fernández-Busnadiego R, Kochanek S. The cryo-electron microscopy structure of huntingtin. Nature. 2018;555:117 - 120. [PMC free article: PMC5837020] [PubMed: 29466333]
- Guo Z, Rudow G, Pletnikova O, Codispoti KE, Orr BA, Crain BJ, Duan W, Margolis RL, Rosenblatt A, Ross CA, Troncoso JC. Striatal neuronal loss correlates with clinical motor impairment in Huntington's disease. Mov Disord. 2012;27:1379 - 86. [PMC free article: PMC3455126] [PubMed: 22975850]
- Gusella JF, Macdonald ME. Huntington's disease: the case for genetic modifiers. Genome Med. 2009;1:80. [PMC free article: PMC2768966] [PubMed: 19725930]
- Hadzi TC, Hendricks AE, Latourelle JC, Lunetta KL, Cupples LA, Gillis T, Mysore JS, Gusella JF, MacDonald ME, Myers RH, Vonsattel JP. Assessment of cortical and striatal involvement in 523 Huntington disease brains. Neurology. 2012;79:1708 - 15. [PMC free article: PMC3468776] [PubMed: 23035064]
- Heinsen H, Rüb U, Bauer M, Ulmar G, Bethke B, Schüler M, Böcker F, Eisenmenger W, Götz M, Korr H, Schmitz C. Nerve cell loss in the thalamic mediodorsal nucleus in Huntington's disease. Acta Neuropathol. 1999;97:613 - 22. [PubMed: 10378380]
- Hendricks AE, Latourelle JC, Lunetta KL, Cupples LA, Wheeler V, Macdonald ME, Gusella JF, Myers RH. Estimating the probability of de novo HD cases from transmissions of expanded penetrant CAG alleles in the Huntington disease gene from male carriers of high normal alleles (27-35 CAG). Am J Med Genet A. 2009;149A:1375 - 81. [PMC free article: PMC2724761] [PubMed: 19507258]
- Hersch SM, Schifitto G, Oakes D, Bredlau AL, Meyers CM, Nahin R, Rosas HD., Huntington Study Group CREST-E Investigators and Coordinators. The CREST-E study of creatine for Huntington disease: a randomized controlled trial. Neurology. 2017;89:594 - 601. [PMC free article: PMC5562960] [PubMed: 28701493]
- Ho AK, Robbins AO, Barker RA. Huntington's disease patients have selective problems with insight. Mov Disord. 2006;21:385 - 9. [PubMed: 16211608]
- Hu J, Liu J, Corey DR. Allele-selective inhibition of huntingtin expression by switching to an miRNA-like RNAi mechanism. Chem Biol. 2010;17:1183 - 8. [PMC free article: PMC3021381] [PubMed: 21095568]
- Huntington Study Group. Effect of deutetrabenazine on chorea among patients with Huntington disease: a randomized clinical trial. JAMA. 2016;316:40 - 50. [PubMed: 27380342]
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology. 2006;66:366 - 72. [PubMed: 16476934]
- Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord. 1996;11:136 - 42. [PubMed: 8684382]
- Huntington Study Group DOMINO Investigators. A futility study of minocycline in Huntington's disease. Mov Disord. 2010;25:2219 - 24. [PubMed: 20721920]
- Huntington Study Group PHAROS Investigators. At risk for Huntington disease: the PHAROS (Prospective Huntington At Risk Observational Study) cohort enrolled. Arch Neurol. 2006;63:991 - 6. [PubMed: 16831969]
- Jama M, Millson A, Miller CE, Lyon E. Triplet repeat primed PCR simplifies testing for Huntington disease. J Mol Diagn. 2013;15:255 - 62. [PubMed: 23414820]
- Jasper MJ, Hu DG, Liebelt J, Sherrin D, Watson R, Tremellen KP, Hussey ND. Singleton births after routine preimplantation genetic diagnosis using exclusion testing (D4S43 and D4S126) for Huntington's disease. Fertil Steril. 2006;85:597 - 602. [PubMed: 16500325]
- Johnson EB, Byrne LM, Gregory S, Rodrigues FB, Blennow K, Durr A, Leavitt BR, Roos RA, Zetterberg H, Tabrizi SJ, Scahill RI, Wild EJ., TRACK-HD Study Group. Neurofilament light protein in blood predicts regional atrophy in Huntington disease. Neurology. 2018;90:e717 - 23. [PMC free article: PMC5818166] [PubMed: 29367444]
- Kalliolia E, Silajdžić E, Nambron R, Hill NR, Doshi A, Frost C, Watt H, Hindmarsh P, Björkqvist M, Warner TT. Plasma melatonin is reduced in Huntington's disease. Mov Disord. 2014;29:1511 - 5. [PubMed: 25164424]
- Kay C, Collins JA, Miedzybrodzka Z, Madore SJ, Gordon ES, Gerry N, Davidson M, Slama RA, Hayden MR. Huntington disease reduced penetrance alleles occur at high frequency in the general population. Neurology. 2016;87:282 - 8. [PMC free article: PMC4955276] [PubMed: 27335115]
- Kay C, Collins JA, Skotte NH, Southwell AL, Warby SC, Caron NS, Doty CN, Nguyen B, Griguoli A, Ross CJ, Squitieri F, Hayden MR. Huntingtin haplotypes provide prioritized target panels for allele-specific silencing in Huntington disease patients of European ancestry. Mol Ther. 2015;23:1759 - 71. [PMC free article: PMC4817952] [PubMed: 26201449]
- Kay C, Collins JA, Wright GEB, Baine F, Miedzybrodzka Z, Aminkeng F, Semaka AJ, McDonald C, Davidson M, Madore SJ, Gordon ES, Gerry NP, Cornejo-Olivas M, Squitieri F, Tishkoff S, Greenberg JL, Krause A, Hayden MR. The molecular epidemiology of Huntington disease is related to intermediate allele frequency and haplotype in the general population. Am J Med Genet B Neuropsychiatr Genet. 2018;177:346 - 57. [PubMed: 29460498]
- Keum JW, Shin A, Gillis T, Mysore JS, Abu Elneel K, Lucente D, Hadzi T, Holmans P, Jones L, Orth M, Kwak S, MacDonald ME, Gusella JF, Lee JM. The HTT CAG-expansion mutation determines age at death but not disease duration in Huntington disease. Am J Hum Genet. 2016;98:287 - 98. [PMC free article: PMC4746370] [PubMed: 26849111]
- Killoran A, Biglan KM. Current therapeutic options for Huntington's disease: good clinical practice versus evidence-based approaches? Mov Disord. 2014;29:1404 - 13. [PubMed: 25164707]
- Killoran A, Biglan KM, Jankovic J, Eberly S, Kayson E, Oakes D, et al. Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS. Neurology. 2013;80:2022 - 7. [PMC free article: PMC3716408] [PubMed: 23624566]
- Kim EH, Thu DC, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, Synek BJ, Waldvogel HJ, Faull RL. Cortical interneuron loss and symptom heterogeneity in Huntington disease. Ann Neurol. 2014;75:717 - 27. [PubMed: 24771513]
- Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, Artates JW, Weiss A, Cheng SH, Shihabuddin LS, Hung G, Bennett CF, Cleveland DW. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031 - 44. [PMC free article: PMC3383626] [PubMed: 22726834]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington's disease. Ann Neurol. 1999;46:842 - 9. [PubMed: 10589536]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR., International Huntington's Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet. 2004;65:267 - 77. [PubMed: 15025718]
- Langbehn DR, Hayden MR, Paulsen JS., PREDICT-HD Investigators of the Huntington Study Group. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:397 - 408. [PMC free article: PMC3048807] [PubMed: 19548255]
- Laplanche JL, Hachimi KH, Durieux I, Thuillet P, Defebvre L, Delasnerie-Laupretre N, Peoc'h K, Foncin JF, Destee A. Prominent psychiatric features and early onset in an inherited prion disease with a new insertional mutation in the prion protein gene. Brain. 1999;122:2375 - 86. [PubMed: 10581230]
- Larsson MU, Luszcz MA, Bui TH, Wahlin TB. Depression and suicidal ideation after predictive testing for Huntington's disease: a two-year follow-up study. J Genet Couns. 2006;15:361 - 74. [PubMed: 16967331]
- Lee JM, Ramos EM, Lee JH, Gillis T, Mysore JS, Hayden MR, Warby SC, Morrison P, Nance M, Ross CA, Margolis RL, Squitieri F, Orobello S, Di Donato S, Gomez-Tortosa E, Ayuso C, Suchowersky O, Trent RJ, McCusker E, Novelletto A, Frontali M, Jones R, Ashizawa T, Frank S, Saint-Hilaire MH, Hersch SM, Rosas HD, Lucente D, Harrison MB, Zanko A, Abramson RK, Marder K, Sequeiros J, Paulsen JS. PREDICT-HD study of the Huntington Study Group (HSG), Landwehrmeyer GB, REGISTRY study of the European Huntington's Disease Network, Myers RH, HD-MAPS Study Group, MacDonald ME, Gusella JF, COHORT study of the HSG. CAG repeat expansion in Huntington disease determines age at onset in a fully dominant fashion. Neurology. 2012;78:690 - 5. [PMC free article: PMC3306163] [PubMed: 22323755]
- Lee JM, Wheeler VC, Chao MJ, Vonsattel JP, Pinto RM, Lucente D, Abu-Elneel K, Ramos EM, Mysore JS, Gillis T, MacDonald ME, Gusella JF, Harold D, Stone TC, Escott-Price V, Han J, Vedernikov A, Holmans P, Jones L, Kwak S, Mahmoudi M, Orth M, Landwehrmeyer GB, Paulsen JS, Dorsey ER, Shoulson I, Myers RH. Identification of genetic factors that modify clinical onset of Huntington's disease. Cell. 2015;162:516 - 26.
- Levin BC, Richie KL, Jakupciak JP. Advances in Huntington's disease diagnostics: development of a standard reference material. Expert Rev Mol Diagn. 2006;6:587 - 96. [PubMed: 16824032]
- Li JL, Hayden MR, Warby SC, Durr A, Morrison PJ, Nance M, Ross CA, Margolis RL, Rosenblatt A, Squitieri F, Frati L, Gomez-Tortosa E, Garcia CA, Suchowersky O, Klimek ML, Trent RJ, McCusker E, Novelletto A, Frontali M, Paulsen JS, Jones R, Ashizawa T, Lazzarini A, Wheeler VC, Prakash R, Xu G, Djoussé L, Mysore JS, Gillis T, Hakky M, Cupples LA, Saint-Hilaire MH, Cha JH, Hersch SM, Penney JB, Harrison MB, Perlman SL, Zanko A, Abramson RK, Lechich AJ, Duckett A, Marder K, Conneally PM, Gusella JF, MacDonald ME, Myers RH. Genome-wide significance for a modifier of age at neurological onset in Huntington's disease at 6q23-24: the HD MAPS study. BMC Med Genet. 2006;7:71. [PMC free article: PMC1586197] [PubMed: 16914060]
- Lin B, Rommens JM, Graham RK, Kalchman M, MacDonald H, Nasir J, Delaney A, Goldberg YP, Hayden MR. Differential 3' polyadenylation of the Huntington disease gene results in two mRNA species with variable tissue expression. Hum Mol Genet. 1993;2:1541 - 5. [PubMed: 7903579]
- Liu D, Long JD, Zhang Y, Raymond LA, Marder K, Rosser A, McCusker EA, Mills JA, Paulsen JS., PREDICT-HD Investigators and Coordinators of the Huntington Study Group. Motor onset and diagnosis in Huntington disease using the diagnostic confidence level. J Neurol. 2015;262:2691 - 8. [PMC free article: PMC4666501] [PubMed: 26410751]
- Lopez WO, Nikkhah G, Schültke E, Furlanetti L, Trippel M. Stereotactic planning software for human neurotransplantation: suitability in 22 surgical cases of Huntington's disease. Restor Neurol Neurosci. 2014;32:259 - 68. [PubMed: 24164802]
- MacDonald ME. Ambrose CM. Duyao MP. Myers RH. Lin C, Srinidhi L. Barnes G, Taylor SA. James M. Groot N. MacFarlane H. Jenkins B. Anderson MA. Wexler NS. Gusella JF. Bates GP, Baxendale S, Hummerich H, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971 - 83.
- MacLeod R, Tibben A, Frontali M, Evers-Kiebooms G, Jones A, Martinez-Descales A, Roos RA. Recommendations for the predictive genetic test in Huntington's disease. Clin Genet. 2013;83:221 - 31. [PubMed: 22642570]
- Majid DS, Aron AR, Thompson W, Sheldon S, Hamza S, Stoffers D, Holland D, Goldstein J, Corey-Bloom J, Dale AM. Basal ganglia atrophy in prodromal Huntington's disease is detectable over one year using automated segmentation. Mov Disord. 2011;26:2544 - 51. [PMC free article: PMC5615846] [PubMed: 21932302]
- Marshall J, White K, Weaver M, Flury Wetherill L, Hui S, Stout JC, Johnson SA, Beristain X, Gray J, Wojcieszek J, Foroud T. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64:116 - 21. [PubMed: 17210818]
- Martino D, Stamelou M, Bhatia KP. The differential diagnosis of Huntington's disease-like syndromes: 'red flags' for the clinician. J Neurol Neurosurg Psychiatry. 2013;84:650 - 6. [PMC free article: PMC3646286] [PubMed: 22993450]
- McBride JL, Pitzer MR, Boudreau RL, Dufour B, Hobbs T, Ojeda SR, Davidson BL. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington's disease. Mol Ther. 2011;19:2152 - 62. [PMC free article: PMC3242667] [PubMed: 22031240]
- McGarry A, McDermott M, Kieburtz K, de Blieck EA, Beal F, Marder K, Ross C, Shoulson I, Gilbert P, Mallonee WM, Guttman M, Wojcieszek J, Kumar R, LeDoux MS, Jenkins M, Rosas HD, Nance M, Biglan K, Como P, Dubinsky RM, Shannon KM, O'Suilleabhain P, Chou K, Walker F, Martin W, Wheelock VL, McCusker E, Jankovic J, Singer C, Sanchez-Ramos J, Scott B, Suchowersky O, Factor SA, Higgins DS Jr, Molho E, Revilla F, Caviness JN, Friedman JH, Perlmutter JS, Feigin A, Anderson K, Rodriguez R, McFarland NR, Margolis RL, Farbman ES, Raymond LA, Suski V, Kostyk S, Colcher A, Seeberger L, Epping E, Esmail S, Diaz N, Fung WL, Diamond A, Frank S, Hanna P, Hermanowicz N, Dure LS, Cudkowicz M. Huntington Study Group 2CARE Investigators and Coordinators. A randomized, double-blind, placebo-controlled trial of coenzyme Q10 in Huntington disease. Neurology. 2017;88:152 - 9. [PMC free article: PMC5224719] [PubMed: 27913695]
- Mehrabi NF, Waldvogel HJ, Tippett LJ, Hogg VM, Synek BJ, Faull RL. Symptom heterogeneity in Huntington's disease correlates with neuronal degeneration in the cerebral cortex. Neurobiol Dis. 2016;96:67 - 74. [PubMed: 27569581]
- Mestre T, Ferreira J, Coelho MM, Rosa M, Sampaio C. Therapeutic interventions for symptomatic treatment in Huntington's disease. Cochrane Database Syst Rev. 2009;3:CD006456. [PubMed: 19588393]
- Michalik A, Van Broeckhoven C. Pathogenesis of polyglutamine disorders: aggregation revisited. Hum Mol Genet. 2003;12(Spec No 2):R173 - 86. [PubMed: 14504263]
- Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington's disease. J Psychiatry Neurosci. 2006;31:21 - 9. [PMC free article: PMC1325063] [PubMed: 16496032]
- Moore RC, Xiang F, Monaghan J, Han D, Zhang Z, Edstrom L, Anvret M, Prusiner SB. Huntington disease phenocopy is a familial prion disease. Am J Hum Genet. 2001;69:1385 - 8. [PMC free article: PMC1235549] [PubMed: 11593450]
- Morton AJ. Circadian and sleep disorder in Huntington's disease. Exp Neurol. 2013;243:34 - 44. [PubMed: 23099415]
- Moss DJH, Pardiñas AF, Langbehn D, Lo K, Leavitt BR, Roos R, Durr A, Mead S, Holmans P, Jones L, Tabrizi SJ, et al. Identification of genetic variants associated with Huntington's disease progression: a genome-wide association study. Lancet Neurol. 2017;16:701 - 11. [PubMed: 28642124]
- Moutou C, Gardes N, Viville S. New tools for preimplantation genetic diagnosis of Huntington's disease and their clinical applications. Eur J Hum Genet. 2004;12:1007 - 14. [PubMed: 15470361]
- Nahhas FA, Garbern J, Krajewski KM, Roa BB, Feldman GL. Juvenile onset Huntington disease resulting from a very large maternal expansion. Am J Med Genet A. 2005;137A:328 - 31. [PubMed: 16096998]
- Nance MA, Myers RH. Juvenile onset Huntington's disease--clinical and research perspectives. Ment Retard Dev Disabil Res Rev. 2001;7:153 - 7. [PubMed: 11553930]
- Okamoto S, Pouladi MA, Talantova M, Yao D, Xia P, Ehrnhoefer D.E, Zaidi R, Clemente A, Kaul M, Graham RK, Zhang D, Chen HS, Tong G, Hayden MR, Lipton SA. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingin. Nat Med. 2009;15:1407 - 13. [PMC free article: PMC2789858] [PubMed: 19915593]
- Ondo WG, Mejia NI, Hunter CB. A pilot study of the clinical efficacy and safety of memantine for Huntington's disease. Parkinsonism Relat Disord. 2007;13:453 - 4. [PubMed: 17046312]
- Palidwor GA, Shcherbinin S, Huska MR, Rasko T, Stelzl U, Arumughan A, Foulle R, Porras P, Sanchez-Pulido L, Wanker EE, Andrade-Navarro MA. Detection of alpha-rod protein repeats using a neural network and application to huntingtin. PLoS Comput Biol. 2009;5:e1000304. [PMC free article: PMC2647740] [PubMed: 19282972]
- Paulsen JS. Functional imaging in Huntington's disease. Exp Neurol. 2009;216:272 - 7. [PMC free article: PMC3810959] [PubMed: 19171138]
- Paulsen JS, Hayden M, Stout JC, Langbehn DR, Aylward E, Ross CA, Guttman M, Nance M, Kieburtz K, Oakes D, Shoulson I, Kayson E, Johnson S, Penziner E. Preparing for preventive clinical trials: the Predict-HD study. Arch Neurol. 2006;63:883 - 90. [PubMed: 16769871]
- Paulsen JS, Hoth KF, Nehl C, Stierman L. Critical periods of suicide risk in Huntington's disease. Am J Psychiatry. 2005a;162:725 - 31. [PubMed: 15800145]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, Duff K, Kayson E, Biglan K, Shoulson I, Oakes D, Hayden M, et al. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874 - 80. [PMC free article: PMC2569211] [PubMed: 18096682]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington's disease. J Neuropsychiatry Clin Neurosci. 2005b;17:496 - 502. [PubMed: 16387989]
- Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington's disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239:11 - 9. [PubMed: 16185716]
- Petersén A, Björkqvist M. Hypothalamic-endocrine aspects in Huntington's disease. Eur J Neurosci. 2006;24:961 - 7. [PubMed: 16925587]
- Petersén A, Gil J, Maat-Schieman ML, Björkqvist M, Tanila H, Araújo IM, Smith R, Popovic N, Wierup N, Norlén P, Li JY, Roos RA, Sundler F, Mulder H, Brundin P. Orexin loss in Huntington's disease. Hum Mol Genet. 2005;14:39 - 47. [PubMed: 15525658]
- Pfister EL, DiNardo N, Mondo E, Borel F, Conroy F, Fraser C, Gernoux G, Han X, Hu D, Johnson E, Kennington L, Liu P, Reid SJ, Sapp E, Vodicka P, Kuchel T, Morton AJ, Howland D, Moser R, Sena-Esteves M, Gao G, Mueller C, DiFiglia M, Aronin N. Artificial miRNAs reduce human mutant huntingtin throughout the striatum in a transgenic sheep model of Huntington's disease. Hum Gene Ther. 2018;29:663 - 73. [PubMed: 29207890]
- Pfister EL, Zamore PD. Huntington's disease: silencing a brutal killer. Exp Neurol. 2009;220:226 - 9. [PMC free article: PMC2805437] [PubMed: 19786020]
- Phillips W, Shannon KM, Barker RA. The current clinical management of Huntington's disease. Mov Disord. 2008;23:1491 - 504. [PubMed: 18581443]
- Potter NT, Spector EB, Prior TW. Technical standards and guidelines for Huntington disease testing. Genet Med. 2004;6:61 - 5. [PubMed: 14726813]
- Pouladi MA, Graham RK, Karasinska JM, Xie Y, Santos RD, Petersén A, Hayden MR. Prevention of depressive behaviour in the YAC128 mouse model of Huntington disease by mutation at residue 586 of huntingtin. Brain. 2009;132:919 - 32. [PubMed: 19224899]
- Pringsheim T, Wiltshire K, Day L, Dykeman J, Steeves T, Jette N. The incidence and prevalence of Huntington's disease: a systematic review and meta-analysis. Mov Disord. 2012;27:1083 - 91. [PubMed: 22692795]
- Pratley RE, Salbe AD, Ravussin E, Caviness JN. Higher sedentary energy expenditure in patients with Huntington's disease. Ann Neurol. 2000;47:64 - 70. [PubMed: 10632102]
- Puri BK, Leavitt BR, Hayden MR, Ross CA, Rosenblatt A, Greenamyre JT, Hersch S, Vaddadi KS, Sword A, Horrobin DF, Manku M, Murck H. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65:286 - 92. [PubMed: 16043801]
- Quarrell OW, Nance MA, Nopoulos P, Paulsen JS, Smith JA, Squitieri F. Managing juvenile Huntington's disease. Neurodegener Dis Manag. 2013:3. [PMC free article: PMC3883192] [PubMed: 24416077]
- Ravina B, Romer M, Constantinescu R, Biglan K, Brocht A, Kieburtz K, Shoulson I, McDermott MP. The relationship between CAG repeat length and clinical progression in Huntington's disease. Mov Disord. 2008;23:1223 - 7. [PubMed: 18512767]
- Rawlins MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, Smeeth L. The prevalence of Huntington's disease. Neuroepidemiology. 2016;46:144 - 53. [PubMed: 26824438]
- Rego AC, de Almeida LP. Molecular targets and therapeutic strategies in Huntington's disease. Curr Drug Targets CNS Neurol Disord. 2005;4:361 - 81. [PubMed: 16101555]
- Reilmann R. Deutetrabenazine-not a revolution but welcome evolution for treating chorea in Huntington disease. JAMA Neurol. 2016;73:1404 - 6. [PubMed: 27749952]
- Reilmann R, Leavitt BR, Ross CA. Diagnostic criteria for Huntington's disease based on natural history. Mov Disord. 2014;29:1335 - 41. [PubMed: 25164527]
- Robbins AO, Ho AK, Barker RA. Weight changes in Huntington's disease. Eur J Neurol. 2006;13:e7. [PubMed: 16879284]
- Robins Wahlin TB. To know or not to know: a review of behaviour and suicidal ideation in preclinical Huntington's disease. Patient Educ Couns. 2007;65:279 - 87. [PubMed: 17000074]
- Robins Wahlin TB, Backman L, Lundin A, Haegermark A, Winblad B, Anvret M. High suicidal ideation in persons testing for Huntington's disease. Acta Neurol Scand. 2000;102:150 - 61. [PubMed: 10987374]
- Romo L, Ashar-Patel A, Pfister E, Aronin N. Alterations in mRNA 3' UTR isoform abundance accompany gene expression changes in human Huntington's disease brains. Cell Rep. 2017;20:3057 - 70. [PMC free article: PMC5625827] [PubMed: 28954224]
- Rosenblatt A. Neuropsychiatry of Huntington's disease. Dialogues Clin Neurosci. 2007;9:191 - 7. [PMC free article: PMC3181855] [PubMed: 17726917]
- Ross CA, Aylward EH, Wild EJ, Langbehn DR, Long JD, Warner JH, Scahill RI, Leavitt BR, Stout JC, Paulsen JS, Reilmann R, Unschuld PG, Wexler A, Margolis RL, Tabrizi SJ. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10:204 - 16. [PubMed: 24614516]
- Rupp J, Blekher T, Jackson J, Beristain X, Marshall J, Hui S, Wojcieszek J, Foroud T. Progression in prediagnostic Huntington disease. J Neurol Neurosurg Psychiatry. 2010;81:379 - 84. [PMC free article: PMC2872788] [PubMed: 19726414]
- Saft C, Lauter T, Kraus PH, Przuntek H, Andrich JE. Dose-dependent improvement of myoclonic hyperkinesia due to Valproic acid in eight Huntington's disease patients: a case series. BMC Neurol. 2006;6:11. [PMC free article: PMC1413552] [PubMed: 16507108]
- Scahill RI, Wild EJ, Tabrizi SJ. Biomarkers for Huntington's disease: an update. Expert Opin Med Diagn. 2012;6:371 - 5. [PubMed: 23480802]
- Schneider SA, Walker RH, Bhatia KP. The Huntington's disease-like syndromes: what to consider in patients with a negative Huntington's disease gene test. Nat Clin Pract Neurol. 2007;3:517 - 25. [PubMed: 17805246]
- Semaka A, Balneaves LG, Hayden MR. "Grasping the grey": patient understanding and interpretation of an intermediate allele predictive test result for Huntington disease. J Genet Couns. 2013a;22:200 - 17. [PubMed: 22903792]
- Semaka A, Creighton S, Warby S, Hayden MR. Predictive testing for Huntington disease: interpretation and significance of intermediate alleles. Clin Genet. 2006;70:283 - 94. [PubMed: 16965319]
- Semaka A, Hayden MR. Evidence-based genetic counselling implications for Huntington disease intermediate allele predictive test results. Clin Genet. 2014;85:303 - 11. [PubMed: 24256063]
- Semaka A, Kay C, Belfroid RD, Bijlsma EK, Losekoot M, van Langen IM, van Maarle MC, Oosterloo M, Hayden MR, van Belzen MJ. A new mutation for Huntington disease following maternal transmission of an intermediate allele. Eur J Med Genet. 2015;58:28 - 30. [PubMed: 25464109]
- Semaka A, Kay C, Doty C, Collins JA, Bijlsma EK, Richards F, Goldberg YP, Hayden MR. CAG size-specific risk estimates for intermediate allele repeat instability in Huntington disease. J Med Genet. 2013b;50:696 - 703. [PubMed: 23896435]
- Semaka A, Kay C, Doty CN, Collins JA, Tam N, Hayden MR. High frequency of intermediate alleles on Huntington disease-associated haplotypes in British Columbia's general population. Am J Med Genet B Neuropsychiatr Genet. 2013c;162B:864 - 71. [PubMed: 24038799]
- Sermon K, De Rijcke M, Lissens W, De Vos A, Platteau P, Bonduelle M, Devroey P, Van Steirteghem A, Liebaers I. Preimplantation genetic diagnosis for Huntington's disease with exclusion testing. Eur J Hum Genet. 2002;10:591 - 8. [PubMed: 12357329]
- Siesling S, van Vugt JP, Zwinderman KA, Kieburtz K, Roos RA. Unified Huntington's disease rating scale: a follow up. Mov Disord. 1998;13:915 - 9. [PubMed: 9827615]
- Singh-Bains MK, Tippett LJ, Hogg VM, Synek BJ, Roxburgh RH, Waldvogel HJ, Faull RL. Globus pallidus degeneration and clinicopathological features of Huntington disease. Ann Neurol. 2016;80:185 - 201. [PubMed: 27255697]
- Sipilä JO, Hietala M, Siitonen A, Päivärinta M, Majamaa K. Epidemiology of Huntington's disease in Finland. Parkinsonism Relat Disord. 2015;21:46 - 9. [PubMed: 25466405]
- Skirton H. Huntington disease: a nursing perspective. Medsurg Nurs. 2005;14:167 - 72. [PubMed: 16035633]
- Skotte NH, Southwell AL, Østergaard ME, Carroll JB, Warby SC, Doty CN, Petoukhov E, Vaid K, Kordasiewicz H, Watt AT, Freier SM, Hung G, Seth PP, Bennett CF, Swayze EE, Hayden MR. Allele-specific suppression of mutant huntingtin using antisense oligonucleotides: providing a therapeutic option for all Huntington disease patients. PLoS One. 2014;9:e107434. [PMC free article: PMC4160241] [PubMed: 25207939]
- Slaughter JR, Martens MP, Slaughter KA. Depression and Huntington's disease: prevalence, clinical manifestations, etiology, and treatment. CNS Spectr. 2001;6:306 - 26. [PubMed: 16113629]
- Slow EJ, Graham RK, Hayden MR. To be or not to be toxic: aggregations in Huntington and Alzheimer disease. Trends Genet. 2006;22:408 - 11. [PubMed: 16806565]
- Slow EJ, Graham RK, Osmand AP, Devon RS, Lu G, Deng Y, Pearson J, Vaid K, Bissada N, Wetzel R, Leavitt BR, Hayden MR. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci U S A. 2005;102:11402 - 7. [PMC free article: PMC1183566] [PubMed: 16076956]
- Southwell AL, Skotte NH, Kordasiewicz HB, Ostergaard ME, Watt AT, Carroll JB, Doty CN, Villanueva EB, Petoukhov E, Vaid K, Xie Y, Freier SM, Swayze EE, Seth PP, Bennett CF, Hayden MR. In vivo evaluation of candidate allele-specific mutant huntingtin gene silencing antisense oligonucleotides. Mol Ther. 2014;22:2093 - 106. [PMC free article: PMC4429695] [PubMed: 25101598]
- Southwell AL, Smith SE, Davis TR, Caron NS, Villanueva EB, Xie Y, Collins JA, Ye ML, Sturrock A, Leavitt BR, Schrum AG, Hayden MR. Ultrasensitive measurement of huntingtin protein in cerebrospinal fluid demonstrates increase with Huntington disease stage and decrease following brain huntingtin suppression. Sci Rep. 2015;5:12166. [PMC free article: PMC4502413] [PubMed: 26174131]
- Squitieri F, Frati L, Ciarmiello A, Lastoria S, Quarrell O. Juvenile Huntington's disease: does a dosage-effect pathogenic mechanism differ from the classical adult disease? Mech Ageing Dev. 2006;127:208 - 12. [PubMed: 16274727]
- Squitieri F, Maglione V, Orobello S, Fornai F. Genotype-, aging-dependent abnormal caspase activity in Huntington disease blood cells. J Neural Transm. 2011;118:1599 - 607. [PubMed: 21519949]
- Stamler D, Bradbury M, Brown F. The pharmacokinetics and safety of deuterated-tetrabenazine. Neurology. 2013;80 Suppl:210.
- Stanek LM, Sardi SP, Mastis B, Richards AR, Treleaven CM, Taksir T, Misra K, Cheng SH, Shihabuddin LS. Silencing mutant huntingtin by adeno-associated virus-mediated RNA interference ameliorates disease manifestations in the YAC128 mouse model of Huntington's disease. Hum Gene Ther. 2014;25:461 - 74. [PMC free article: PMC4028091] [PubMed: 24484067]
- Stern HJ, Harton GL, Sisson ME, Jones SL, Fallon LA, Thorsell LP, Getlinger ME, Black SH, Schulman JD. Non-disclosing preimplantation genetic diagnosis for Huntington disease. Prenat Diagn. 2002;22:503 - 7. [PubMed: 12116316]
- Stoy N, McKay E. Weight loss in Huntington's disease. Ann Neurol. 2000;48:130 - 1. [PubMed: 10894233]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC., TRACK-HD investigators. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8:791 - 801. [PMC free article: PMC3725974] [PubMed: 19646924]
- Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, Jones R, Johnson H, Craufurd D, Hicks SL, Kennard C, Landwehrmeyer B, Stout JC, Borowsky B, Scahill RI, Frost C, Langbehn DR., TRACK-HD investigators. Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11:42 - 53. [PubMed: 22137354]
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, Kennard C, Craufurd D, Frost C, Langbehn DR, Reilmann R, Stout JC. TRACK-HD Investigators. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10:31 - 42. [PubMed: 21130037]
- Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, Borowsky B, Landwehrmeyer B, Frost C, Johnson H, Craufurd D, Reilmann R, Stout JC, Langbehn DR. TRACK-HD Investigators. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637 - 49. [PubMed: 23664844]
- Taylor S. Gender differences in attitudes among those at risk for Huntington's disease. Genet Test. 2005;9:152 - 7. [PubMed: 15943556]
- Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, Luthi-Carter R, Waldvogel HJ, Faull RL. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 2010;133(Pt 4):1094 - 110. [PubMed: 20375136]
- Timman R, Claus H, Slingerland H, van der Schalk M, Demeulenaere S, Roos RA, Tibben A. Nature and development of Huntington disease in a nursing home population: The Behavior Observation Scale Huntington (BOSH). Cogn Behav Neurol. 2005;18:215 - 22. [PubMed: 16340395]
- Trejo A, Tarrats RM, Alonso ME, Boll MC, Ochoa A, Velásquez L. Assessment of the nutrition status of patients with Huntington's disease. Nutrition. 2004;20:192 - 6. [PubMed: 14962685]
- Valenza M, Cattaneo E. Cholesterol dysfunction in neurodegenerative diseases: Is Huntington's disease in the list? Prog Neurobiol. 2006;80:165 - 76. [PubMed: 17067733]
- van der Burg JM, Björkqvist M, Brundin P. Beyond the brain: widespread pathology in Huntington's disease. Lancet Neurol. 2009;8:765 - 74. [PubMed: 19608102]
- van der Burg JMM, Gardiner SL, Ludolph AC, Landwehrmeyer GB, Roos RAC, Aziz NA. Body weight is a robust predictor of clinical progression in Huntington disease. Ann Neurol. 2017;82:479 - 83. [PubMed: 28779551]
- van Duijn E, Vrijmoeth EM, Giltay EJ, Bernhard Landwehrmeyer G, REGISTRY investigators of the European Huntington's Disease Network. Suicidal ideation and suicidal behavior according to the C-SSRS in a European cohort of Huntington's disease gene expansion carriers. J Affect Disord. 2018;228:194-204. [PubMed: 29253686]
- Videnovic A, Leurgans S, Fan W, Jaglin J, Shannon KM. Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat Disord. 2009;15:471 - 4. [PubMed: 19041273]
- Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369 - 84. [PubMed: 9596408]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP Jr. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559 - 77. [PubMed: 2932539]
- Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The neuropathology of Huntington's disease. Curr Top Behav Neurosci. 2015;22:33 - 80. [PubMed: 25300927]
- Wang R, Ross CA, Cai H, Cong WN, Daimon CM, Carlson OD, Egan JM, Siddiqui S, Maudsley S, Martin B. Metabolic and hormonal signatures in pre-manifest and manifest Huntington's disease patients. Front Physiol. 2014;5:231. [PMC free article: PMC4066441] [PubMed: 25002850]
- Warby SC, Montpetit A, Hayden AR, Carroll JB, Butland SL, Visscher H, Collins JA, Semaka A, Hudson TJ, Hayden MR. CAG expansion in the Huntington disease gene is associated with a specific and targetable predisposing haplogroup. Am J Hum Genet. 2009;84:351 - 66. [PMC free article: PMC2668007] [PubMed: 19249009]
- Warby SC, Visscher H, Collins JA, Doty CN, Carter C, Butland SL, Hayden AR, Kanazawa I, Ross CJ, Hayden MR. HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet. 2011;19:561 - 6. [PMC free article: PMC3083615] [PubMed: 21248742]
- Wexler NS, Lorimer J, Porter J, Gomez F, Moskowitz C, Shackell E, Marder K, Penchaszadeh G, Roberts SA, Gayan J, Brocklebank D, Cherny SS, Cardon LR, Gray J, Dlouhy SR, Wiktorski S, Hodes ME, Conneally PM, Penney JB, Gusella J, Cha JH, Irizarry M, Rosas D, Hersch S, Hollingsworth Z, MacDonald M, Young AB, Andresen JM, Housman DE, De Young MM, Bonilla E, Stillings T, Negrette A, Snodgrass SR, Martinez-Jaurrieta MD, Ramos-Arroyo MA, Bickham J, Ramos JS, Marshall F, Shoulson I, Rey GJ, Feigin A, Arnheim N, Acevedo-Cruz A, Acosta L, Alvir J, Fischbeck K, Thompson LM, Young A, Dure L, O'Brien CJ, Paulsen J, Brickman A, Krch D, Peery S, Hogarth P, Higgins DS Jr, Landwehrmeyer B. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proc Natl Acad Sci U S A. 2004;101:3498 - 503. [PMC free article: PMC373491] [PubMed: 14993615]
- Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S, Miller JR, Zetterberg H, Leavitt BR, Kuhn R, Tabrizi SJ, Macdonald D, Weiss A. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington's disease patients. J Clin Invest. 2015;125:1979 - 86. [PMC free article: PMC4463213] [PubMed: 25844897]
- Wild EJ, Tabrizi SJ. Targets for future clinical trials in Huntington's disease: What's in the pipeline? Mov Disord. 2014;29:1434 - 45. [PMC free article: PMC4265300] [PubMed: 25155142]
- Williams JK, Skirton H, Paulsen JS, Tripp-Reimer T, Jarmon L, McGonigal Kenney M, Birrer E, Hennig BL, Honeyford J. The emotional experiences of family carers in Huntington disease. J Adv Nurs. 2009;65:789 - 98. [PMC free article: PMC3807601] [PubMed: 19228233]
- Xu M, Wu ZY. Huntington disease in Asia. Chin Med J (Engl). 2015;128:1815 - 9. [PMC free article: PMC4733723] [PubMed: 26112725]
- Yoon G, Kramer J, Zanko A, Guzijan M, Lin S, Foster-Barber A, Boxer AL. Speech and language delay are early manifestations of juvenile-onset Huntington disease. Neurology. 2006;67:1265 - 7. [PubMed: 17030763]
- Youssov K, Dolbeau G, Maison P, Boissé M-F, Cleret de Langavant L, Roos RAC, et al. Unified Huntington's disease rating scale for advanced patients: validation and follow-up study. Mov Disord. 2013;28:1717 - 23. [PMC free article: PMC5071381] [PubMed: 24166899]